Effective Guide to How to Draw Lewis Structures: Unveiling Current Techniques for 2025
Effective Guide to How to Draw Lewis Structures
Understanding how to draw Lewis structures is essential for mastering **chemical bonding** and molecular representations in chemistry. A **Lewis structure** represents the arrangement of atoms in a molecule and illustrates how **valence electrons** are distributed among the atoms. This guide aims to unveil techniques and rules for drawing accurate Lewis structures, ushering in a better understanding of their role in predicting **molecular geometry** and chemical behavior. As we look towards 2025, these modern methods and resources will be invaluable for students, educators, and professionals alike.
Understanding Basic Concepts of Lewis Structures
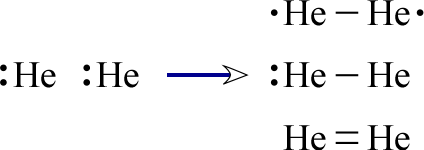
Before diving into methodologies for drawing Lewis structures, it is crucial to familiarize yourself with some fundamental concepts such as **valence electrons**, **octet rule**, and the types of bonds that can form such as **covalent bonds**. **Valence electrons** are the outermost electrons of an atom, responsible for chemical bonding. According to the **octet rule**, atoms tend to bond in such a way that they achieve a full complement of eight electrons in their outer shell, resembling the electron configuration of noble gases. In creating **Lewis structures**, understanding how **electron dot diagrams** illustrate these electrons helps visualize bonding and lone pairs more clearly.
Valence Electrons and the Octet Rule
The first step in drawing a Lewis structure involves calculating the total number of **valence electrons** available in a molecule. For instance, if you are working with **carbon dioxide (CO2)**, carbon (C) has four valence electrons and each oxygen (O) has six, leading to a total of 16 valence electrons. These electrons then must be arranged to adhere to the **octet rule**, with each atom (except hydrogen, which follows the duet rule) surrounded by eight electrons in the form of bonds and lone pairs. Recognizing this balance brings clarity in the structure’s representation and understanding of predicted **molecular geometry**.
Covalent Bonds and Electron Sharing
Next, familiarize yourself with the types of bonds that can be formed. **Covalent bonds** occur when two atoms share electrons, typically to satisfy the **octet rule**. There are various classifications of covalent bonds: single bonds, double bonds, and triple bonds. For example, **methane (CH4)** has four single bonds formed by the sharing of electrons between carbon and hydrogen atoms. Understanding the strength and behavior of these bonds is fundamental when constructing accurate Lewis structures.
Common Mistakes in Drawing Lewis Structures
A diploma in drawing **Lewis structures** often involves learning from common mistakes made during the process. Miscounting **valence electrons** and misunderstanding **bonding pair** arrangements can lead to flawed structures. Often, beginners may not recognize the necessity of including **lone pairs**; neglecting these electron pairs can misrepresent the true stability and structure of the molecule. Therefore, increasing awareness of these potential pitfalls is integral to mastering this skill.
Counting Valence Electrons Accurately
One prevalent mistake when developing Lewis structures is miscalculating the total number of **valence electrons**. To mitigate this, it is prudent to follow a systematic approach: first, check the group number of an atom in the periodic table. Each atom’s group number corresponds to the number of valence electrons. Moreover, pay close attention when dealing with ions; for negatively charged ions, add the charge’s numerical value to the total electron count, whereas for positively charged ions, subtract it. This approach guarantees an accurate electron count and enhances the integrity of your Lewis structures.
Including Lone Pairs and Understanding Formal Charge
Another common error in crafting Lewis structures is the omission of **lone pairs**. Lone pairs are essential as they represent unshared valence electrons that play a crucial role in determining molecular shape and behavior during reactions. Furthermore, once you establish the bonds and lone pairs, calculating the **formal charge** helps ensure the stability of your structure. The formal charge indicates how well the electrons are allocated to each atom compared to their neutral state. Ideally, you want the formal charge to be close to zero across the molecule for it to be the most stable.
Advanced Techniques in Drawing Lewis Structures
As you become comfortable with the basics of **Lewis structures**, advancing your techniques to accommodate larger and more complex molecules becomes necessary. This means dealing with structures that abide by the **octet expansion** rule or showcasing **resonance structures** that reveal a molecule’s true nature through various possible configurations.
Understanding Resonance Structures
Many molecules do not resonate with just a single Lewis structure; instead, they often adopt multiple valid representations. For instance, ozone (O3) is best represented by two resonance structures that equilibrate bond character and charge distribution among the atoms. Understanding when to represent a molecule by multiple **resonance structures** is critical for advanced chemistry realms as it conveys the true nature of certain bonds and some molecular features that an individual Lewis structure may not accurately illustrate.
Applying the Octet Expansion Rule
When drawing complex molecules, especially those beyond the second period of the periodic table, such as phosphorus or sulfur compounds, you might encounter cases where **octet expansion** is necessary. Such elements can accommodate more than 8 electrons around them due to the availability of d-orbitals. An illustrative example is **phosphorus pentachloride (PCl5)**, where the phosphorus atom shares electrons with five chlorine atoms, resulting in a stable structure even as it exceeds the octet rule. Recognizing structures that require octet expansion is vital in accurately depicting larger molecules.
Learning and Visualization Techniques
In 2025 and beyond, educational technologies provide students and researchers with valuable resources to enhance their understanding of **Lewis structures** and **chemical bonding**. Utilizing **visual aids** like **3D visualization** software enables clearer comprehension of the spatial orientations of bonds and molecular shapes. Our engagement with such technologies is essential for grasping modern chemical concepts.
Utilizing Software for 3D Visualization
There are numerous applications available today that allow students to visualize molecules’ 3D representations, enhancing the learning experience about Lewis structures and their corresponding molecular geometries. For instance, software such as Avogadro or ChemDraw can demonstrate how bond angles and lengths change within molecules, accommodating various functional groups. Understanding this functionality is essential for advanced learners and professionals applying chemistry in practical environments such as laboratories or industries.
Interactive Learning Tools for Improved Engagement
Moreover, utilizing **interactive learning tools** online fosters collaborative learning while making it possible to tackle increasingly complex information concerning **Lewis structures**. Utilizing video tutorials with demonstrative examples, engaging in problem-solving exercises, and joining peer study groups increases retention of knowledge and builds foundational skills in chemistry. Such methods empower learners to appreciate the underlying principles beyond rote memorization, promoting critical thinking and effective application.
Key Takeaways
- Accurate counting of valence electrons is fundamental for drawing reliable Lewis structures.
- Understanding bonding types (single, double, triple) is crucial in illustrating the correct arrangements of atoms.
- Recognizing the role of lone pairs and formal charges helps depict stable molecular identities.
- Advanced techniques, including resonance structures and octet expansion, are vital for complex molecules.
- Embracing modern technologies and interactive learning tools can significantly enhance engagement and comprehension.
FAQ
1. What is the significance of using Lewis structures in chemistry?
Lewis structures act as a fundamental tool for visualizing molecular structure, illustrating **electron sharing**, and predicting chemical reactivity. By representing the distribution of **valence electrons**, these diagrams help chemists acquire insights into how molecules interact via **bonding** and their **molecular shapes**, essential in understanding chemical properties and reactions.
2. How do resonance structures enhance our understanding of certain molecules?
Resonance structures allow chemists to illustrate a molecule’s behavior where a single structure cannot fully capture the compound’s characteristics. They show that the actual structure is a hybrid of these different forms. For instance, resonance in benzene demonstrates its **stability** and uniform bond lengths, showcasing the delocalization of electrons.
3. What is the octet rule, and when might it be exceeded?
The **octet rule** states that atoms tend to form bonds in such a way as to have eight electrons in their valence shell, resembling noble gas configurations. However, atoms in the third period and lower, such as phosphorus and sulfur, can accommodate more than eight electrons due to the availability of d-orbitals, resulting in **octet expansion** in complex molecules.
4. What common mistakes should be avoided when drafting Lewis structures?
Common pitfalls include miscounting **valence electrons** and neglecting **lone pairs** in the structure. Additionally, underestimating formal charges can lead to unstable or incorrect representations. Being vigilant about these aspects ensures more precise chemical drawings.
5. How can modern visualization techniques aid in learning about Lewis structures?
Modern visualization techniques, such as **3D visualization software**, allow students and chemists to see molecular shapes and bond angles in a more interactive way. This representation enhances understanding and retention of complex 3D arrangements and their related chemistry, making it easier to grasp bonding concepts.
6. Why are resonance structures important for assessing stability in larger molecules?
Resonance structures provide insights into how electron distribution occurs in larger molecules, impacting their overall **stability**. They reveal possible configurations that acknowledge bond character and charge spread over atoms, allowing a better understanding of a molecule’s reactivity or interactions, particularly in the context of **chemical equilibrium** and reactions.
7. What strategies can be used for effectively teaching Lewis structures?
Employing interactive tasks, hands-on activities, and collaborative peer-learning can significantly bolster learning outcomes when teaching **Lewis structures**. Additionally, the use of visual aids and **mnemonic devices** helps in recalling complex bond patterns effectively, catering to diverse learning styles in educational settings.